

Here at Genesis Research Services, we conduct a large number of clinical trials for new medical devices, as well as pharmaceutical / drug trials. In general, the approach to testing devices is fairly similar to testing new drugs – there is a need for preclinical research, there are strict regulations, safety and ethical requirements, and the testing process is broken up into a series of phases/stages. Our previous article, “Introduction to Clinical Trials”, provides an overview of these topics and more. There are, however, some obvious differences when conducting clinical trials for medical devices, compared to pharmaceutical trials, which we will describe here.
By definition, a medical device is any “article, instrument, apparatus or machine that is used in the prevention, diagnosis or treatment of illness or disease, or for detecting, measuring, restoring, correcting or modifying the structure or function of the body for some health purpose” (World Health Organisation). This includes something a simple as an adhesive bandage, artificial body parts (prostheses), any surgical, diagnostic or monitoring equipment/devices, as well as advanced, implanted devices such as pacemakers and neurostimulators.
Medical devices are classified based on their intended use, invasiveness, duration of use, and the risks and potential harms associated with their use. The following table provides an overview of the classes defined by the Therapeutic Goods Administration (TGA) Australia:
Class | Risk | Clinical Trials? | Examples |
I | Minimal | No | · Adhesive bandages · Crutches · Tongue depressors |
IIa | Low to moderate | Maybe | · Hypodermic needles · Gauze dressings · TENS devices |
IIb | Moderate to high | Maybe | · Lung ventilators · Insulin pens · Diagnostic X-rays |
III | High | Yes | · Heart valves · Spinal & vascular stents |
AIMD | High (Active Implantable Medical Devices) | Yes | · Pacemakers · Spinal cord stimulators · Defibrillators |
Not all medical devices will require clinical trials before they may be approved for market release. Class I devices are such low risk that they don’t require clinical trials. In Australia, however, all medical devices regardless of their class must comply with the Australian Regulatory Guidelines for Medical Devices (ARGMD), which includes requirements for device design and manufacturing, benefits that outweigh the risks, minimisation of risks and unwanted effects, and so forth. With the exception of Class I devices, they must also be registered with the Australian Register of Therapeutic Goods (ARTG). The approval process may require an audit and clinical evidence or data. Whether a device needs to undergo clinical trials relies on its class, whether it is substantially similar to or based on an already approved device, and/or whether significant clinical data to support the new device has already been published in the medical literature or not.
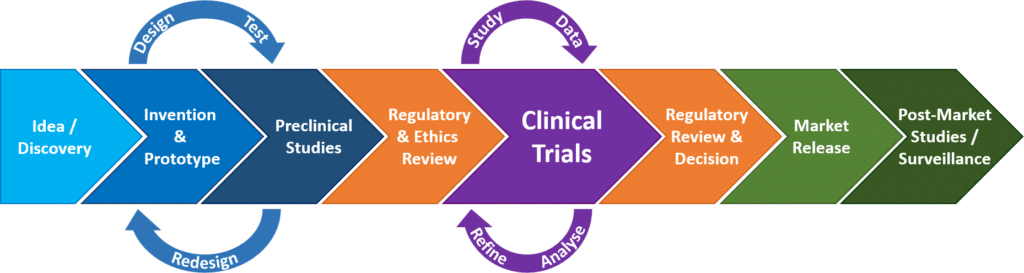
The idea for a new medical device usually comes from the mind of a physician and/or an engineer, as opposed to a new drug, which usually comes from basic laboratory research. If the idea is determined to be workable and practical (proof of concept) an early design of the device, known as a prototype, will be built.
A prototype device will undergo a cycle of preclinical testing -> redesigning -> preclinical testing of the redesign -> and so forth, until the design has been refined and tested to a point that it is ready for production and testing in humans. Preclinical testing may involve the following:
Before a clinical trial involving a new device (or drug) may begin, the proposed study and device must undergo a review and notification process for the purpose of ensuring the safety of study participants:
In our previous article, “Introduction to Clinical Trials”, we talked about how clinical research is broken up into a series of “phases”, each with a different distinct purpose, beginning with (though not always conducted) pilot studies, then safety testing, then efficacy testing, then clinical efficacy, and, if approved for market release, post-market monitoring. New pharmaceuticals/drugs all undergo this clinical trial phase sequence. For medical devices, the sequence is fairly similar, and some devices do go through a clinical trial phase process, however, most medical devices will go through clinical trial “stages”, instead of phases. The table below provides a side by side comparison of pharmaceutical trial phases versus medical device trial stages.
Pharmaceuticals | Medical Devices | ||||
Phase | Subjects | Purpose | Stage | Subjects | Purpose |
0 – Pilot / Exploratory | 10 – 15 | · Test a very small (subtherapeutic) dose of a new drug to study its effects & how it works in the human body. · Not all drugs will undergo this phase. | Pilot / Early Feasibility / First-in-Human | 10 – 30 | · Small study to collect preliminary safety & device performance data in humans. · Guides device modifications &/or future study design. |
I – Safety & Toxicity | 10 – 100 | · True first-in-human study to test safety & toxicity, usually in healthy humans. | Traditional Feasibility | 20 – 30 | · Assess safety & efficacy of the near-final or final device design in patients. · Guides the design of the pivotal study. |
II – Safety & Efficacy | 100’s | · Assess efficacy & safety in patients. | |||
III – Clinical Effectiveness | 100’s – 1000’s | · Confirm clinical efficacy, safety & adverse events. · Compare the new drug to standard care or a commonly used drug. | Pivotal | 100’s | · Large study to confirm clinical efficacy, safety & risks. · Statistically driven. |
IV – Post-Market / Surveillance | 1000’s | · Monitor long term effectiveness & safety in the general population. | Post-Market | 1000’s | · Monitor long term effectiveness, safety & usage in the general population. |
The gold standard of study design for clinical trials is a randomised controlled trial, preferably with blinding and a placebo as the control. To recap on this terminology:
This study design is often much more difficult or not possible to implement for medical devices, however. A well-controlled study design is generally acceptable. The table below compares study design elements between pharmaceutical and medical device trials:
Pharmaceutical Trials | Medical Device Trials |
· Controlled studies o E.g. drug vs no drug o Or, vs commonly used drug or standard care · Placebos used o E.g. sugar pills, saline injection · Randomisation · Blinding (single / double-blinded) · Crossover studies | · Controlled studies o E.g. device vs no device o Or, vs another device, standard care or physical therapy · Placebos rare o E.g. sham surgery, inactive device · Sometimes randomised · Blinding is rare / difficult · Crossover studies sometimes possible |
There is a lot of controversy surrounding the use of a placebo for surgical / implanted devices because the standard placebo would be a sham surgery or implantation of a sham device. Sham procedures are high risk and may be considered unethical. Without this kind of control, however, there is no sure way of knowing whether the device is providing real clinical benefit or if the benefit experienced is due to the placebo effect. It is the job of the ethics committee to determine if the risks associated with a sham surgery outweigh the risk of mistaking clinical benefit for a placebo effect.
For implanted devices that don’t induce any physical sensations (e.g. neurostimulators that don’t produce paraesthesia / “pins and needles”), the issue of a placebo may be balanced by implementing a crossover study design. An example would be a study in which all patients receive the implanted device, however, the patients in the control group don’t have their device switched on (activated) until the crossover point, at which point device activation/deactivation would be reversed between the groups. This kind of study could be blinded, although double-blinding may be difficult as someone would have to activate/deactivate the devices.
Along with the standard list of health professionals involved in clinical trials, such as the study doctor(s) / investigator(s) and clinical trial coordinator(s), patients in medical device trials will usually see the following additional health professionals:
For trials involving implanted devices or devices that require regular check-ups, maintenance and/or programming, patients will typically be seen by a representative of the device company. This person may also be known as a technician or programmer. They will usually be present at the implant surgery and will meet with the patient during their post-op visit to check on and activate the device.
For neurostimulators and other programmable devices, the programmer will spend time with the patient while they tweak the device’s program settings in order to suit the patient’s individual needs. There is rarely a one-size fits all approach to programmable devices, and so this visit may take much longer than standard study visits. Multiple programming visits are often required, whether on a regular or an as-needed basis, in order for the programmer to optimise the device and address any potential unwanted effects or adverse events.
For surgical/implanted devices, patients will be seen by a nurse. Advanced devices such as neurostimulators may also require a patient education session, which may be led by a nurse. Nurses may perform post-operative wound checks and phone calls to check on patients, however, the study coordinator will remain the primary contact person for study patients.
Many device trials will involve medical imaging staff, either for screening / diagnostic purposes (including x-rays, CT-scans, MRI scans) and/or to check the anatomical placement of an implanted device. Other health professionals that may be involved include pathology staff (i.e. for blood tests), psychologists, physiotherapists, and so forth, depending on the device and trial design.
1. Australian Clinical Trials: https://www.australianclinicaltrials.gov.au
2. ClinicalTrials.gov (NIH: U.S. National Library of Medicine): https://clinicaltrials.gov
3. Medical Technology Association of Australia (MTAA): https://www.mtaa.org.au/
a. “Clinical Trials for Medical Devices – The Basics”: https://www.mtaa.org.au/clinical-trials-medical-devices-basics
4. U.S. Food and Drug Administration (FDA): https://www.fda.gov/
a. “Medical Devices”: https://www.fda.gov/MedicalDevices/default.htm
b. “Learn About Drug and Device Approvals”. January 2018: https://www.fda.gov/ForPatients/Approvals/default.htm
5. Therapeutic Goods Administration (TGA): https://www.tga.gov.au
a. “Australian clinical trial handbook”. March 2018: https://www.tga.gov.au/book/export/html/5263
b. “Australian regulatory guidelines for medical devices (ARGMD)” Version 1.1; May 2011: https://www.tga.gov.au/publication/australian-regulatory-guidelines-medical-devices-argmd
c. “Clinical Trials”. April 2018: https://www.tga.gov.au/clinical-trials
d. “Medical devices regulation: an introduction”. June 2017: https://www.tga.gov.au/sme-assist/medical-devices-regulation-introduction
e. “Guidance on conduction clinical trials in Australia using ‘unapproved’ therapeutic goods: The CTN and CTX schemes”. March 2018: https://www.tga.gov.au/book-page/ctn-and-ctx-schemes
6. World Health Organisation: “Medical devices”: http://www.who.int/medical_devices/full_deffinition/en/
7. Institute of Medicine (US) Committee on Technological Innovation in Medicine; Gelijns, AC, editor. “Appendix A: Comparing the Development of Drugs, Devices, and Clinical Procedures” In: Modern Methods of Clinical Investigation: Medical Innovation at the Crossroads: Volume I. Washington (DC): National Academies Press (US); 1990: https://www.ncbi.nlm.nih.gov/books/NBK235489/
8. Neuroscience Trials Australia; “CTN AND CTX SCHEMES SMOOTH THE WAY FOR CLINICAL TRIALS IN AUSTRALIA”. October 2017: http://www.neurotrialsaustralia.com/ctn-and-ctx-schemes-smooth-the-way-for-clinical-trials-in-australia/
9. Kaplan, AV, Baim, DS, Smith JJ, et al. “Medical Device Development: From Prototype to Regulatory Approval”. Circulation 2004;109(25):3068-3072: http://circ.ahajournals.org/content/109/25/3068
10. Redberg. RF. “Sham Controls in Medical Device Trials”. The New England Journal of Medicine 2014;371(10):892-3: https://www.nejm.org/doi/10.1056/NEJMp1406388
11. Van Norman, GA. “Drugs, Devices, and the FDA: Part 2: An Overview of Approval Processes: FDA Approval of Medical Devices”. JACC: Basic to Translational Science 2016;1(4):277-287: https://www.sciencedirect.com/science/article/pii/S2452302X16300183
12. Viceconti, M, Henney, A, Morley-Fletcher, E. “In silico clinical trials: how computer simulation will transform the biomedical industry”. International Journal of Clinical Trials 2016;3(2):37-46: http://www.ijclinicaltrials.com/index.php/ijct/article/view/105
View our currently recruiting studies:
Register your interest for future studies:
© Genesis Research Services, 2023